The various smelting and refining
reactions involved in the iron and steelmaking processes generally
proceed at high temperatures, so there are few cases where the
chemical reaction rate controls the overall reaction rate. For
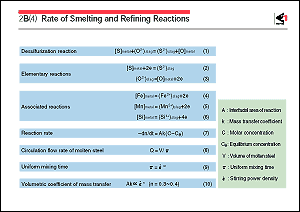
example, the desulfurization of molten steel by slag shown in
Eq. 1 is composed of the elementary reactions given by Eqs. 2
and 3 and the associated reactions given by Eqs. 4, 5 and 6.
It is known that these elementary and associated reactions are
rate-controlled by mass transfer of the reactants in less than
1 second from the start of the reactions. As with decarburization,
desulfurization and dephosphorization, when the overall reaction
is rate-controlled by the reactant's mass transfer to the reaction
interface, the change in the reactant's mole number "n"
after time "t", i.e., the reaction rate "-dn/dt",
can be expressed by Eq.7 as a function of area "A"
of the reaction interface, mass transfer rate constant "k",
the reactant's molar concentration "C", and equilibrium
concentration "Ce".
To increase the rate "-dn/dt" of the overall reaction
(the overall mass transfer rate), it is necessary to make "Ak"
(the volumetric coefficient of mass transfer) large and "Ce"
small. The conditions for making "Ce" small are determined
by a thermodynamic consideration. For any reaction of a gas/metal
system or a slag/metal system, "Ak" can be made very
large by blowing gas or slag into the metal bath or by enhancing
the stirred flow and intermixing the slag and metal. The area
"A" of the reaction interface increases markedly when
gas is blown into the metal bath in a gas/metal system, and slag
is blown into the metal bath (or metal is blown into the slag
bath) in a slag/metal system; in such cases, the constant "k"
also increases at the same time because of the stirring of slag
or metal which accelerates the transfer of the reactants to the
reaction interface.
Taking a molten steel bath of volume "V" as an example,
it is conceivable that the rate at which the reactants in the
bath reach the reaction interface is proportional to circulating
flow rate "Q" of the molten steel in the bath. The
rate "Q" is related by Eq. 8 to the uniform mixing
time " ", which refers to the time necessary
for the reactants to disperse uniformly in the bath. Time " ", which refers to the time necessary
for the reactants to disperse uniformly in the bath. Time " " is related by Eq. 9 to the stirring power density
" " is related by Eq. 9 to the stirring power density
"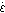 ", which refers to the power of stirring applied
per unit weight or volume of the bath. In the refining reactions
for a gas/metal system and slag/metal system, there are many
cases in which Eq. 10 can be applied, even when the power for
stirring is applied in different manners, such as by gas blowing,
electromagnetic induction, or mechanical rotation. The reason
why "n" takes the value of 0.3-0.4 has been explained
for the respective stirring method in terms of transport phenomena.
However, "n" may sometimes reach 0.4-1.0 when " ", which refers to the power of stirring applied
per unit weight or volume of the bath. In the refining reactions
for a gas/metal system and slag/metal system, there are many
cases in which Eq. 10 can be applied, even when the power for
stirring is applied in different manners, such as by gas blowing,
electromagnetic induction, or mechanical rotation. The reason
why "n" takes the value of 0.3-0.4 has been explained
for the respective stirring method in terms of transport phenomena.
However, "n" may sometimes reach 0.4-1.0 when "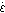 " increases further and metal particles disperse
into the gas or slag, or gas bubbles and slag particles disperse
into metal. " increases further and metal particles disperse
into the gas or slag, or gas bubbles and slag particles disperse
into metal.
As in the example for the rate of ore reduction by gas flowing
through the charged layers of coke and ore in a BF, research
on the solid phase reaction rate has also progressed to such
an extent that ore reduction can be treated as the problem of
a migrating boundary layer, and analysis is made by linking the
ascent of the gas with the heat and mass transfer caused by the
descent of lump ore and coke into the lower shaft of the BF. |
|
 |
 |
 |
|
|