Ordinary carbon steels tend to
corrode in air. The corrosion products from ordinary carbon steels
in air are a cohesive mixture of fine particles of FeOOH[=1/2(Fe2O3・H2O)]
and Fe3O4.
Corrosion occurs by the combined effect of water and oxygen and
continues as long as water and oxygen are both supplied. Therefore,
corrosion can be suppressed or prevented by avoiding water and
oxygen from direct contact with the surface of a steel sheet.
Painting and chemical treatment are carried out to achieve this.
The ultimate target for steel materials used in air, with regard
to corrosion resistance, is to use them in the unpainted condition
without excessive alloying of Ni and/or Cr. The materials developed
for this purpose are referred to as atmospheric corrosion-resistant
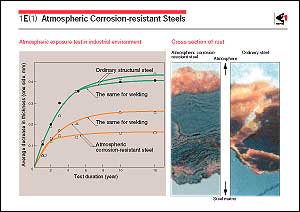
steels (weathering steels). The figure shows the thickness losses
of steels caused by corrosion in air. The corrosion loss of atmospheric
corrosion-resistant steel is only half that of ordinary carbon
steel. The photograph shows a comparison of the cross section
of rust formed in air between ordinary carbon steel and atmospheric
corrosion-resistant steel. The black and gray parts on the base
metal are amorphous layers of enriched chromium and copper, while
the yellow parts are crystalline rust layers. The whole surface
of atmospheric corrosion-resistant steel is covered with an amorphous
layer that provides high corrosion resistance. It is believed
that this amorphous layer suppresses the progress of corrosion
by forming stabilized rust, and preventing oxygen and water penetrating
through the rust. However, neither the detailed structure of
stabilized rust nor its formation mechanism has yet been thoroughly
clarified.
The use of unpainted atmospheric corrosion-resistant steels is
spreading for buildings and bridges to be constructed in inland
areas. It is expected that their area of application will be
extended to coastal areas by further improvements in their characteristics
through future research and development. |
|
 |
 |
 |
|
|