The chemical reactions occurring
in metallurgical processes such as the reduction of iron ore
and the decarburization of molten iron can be explained in terms
of the free energy in thermodynamics. When the free energy of
reactants is different from that of products, a reaction will
occur. If it leads to a decrease in free energy, it will reach
equilibrium when the free energy of the reactants and products
becomes equal. For an explanation of the smelting and refining
reaction, Gibb's energy (free energy at constant pressure) is
used, which is calculated by using temperature, pressure, and
concentration as independent variables. The reactions for smelting
and refining are based on those of reduction and oxidation.
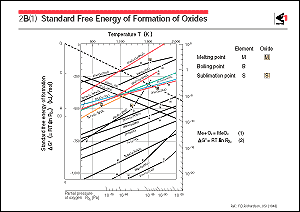
The figure shows the changes in the standard Gibb's energy which
occur when oxides of elements are formed by the chemical reactions
for producing iron and steel. This figure is called the Ellingham
diagram. In general, the reaction in which an element reacts
with 1 mole of oxygen to form an oxide is given by Eq. 1, and
the change in the standard Gibb's energy of this reaction is
given by Eq. 2, where 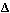 G0 is called the oxygen
potential. G0 is called the oxygen
potential.
Taking an example of the reaction in which magnetite (Fe3O4)
combines with oxygen to form hematite (Fe2O3), the Gibb's energy
change at 1,000K during this reaction is about 200 kilojoules
per 1 mole from the value at point A. When the straight line
that connects point A with point Q is extrapolated to the line
outside the right-hand margin, these two lines cross at a point
where the partial pressure of oxygen (Po2) is about 10-6 Pascals
(Pa). This shows that the reaction reaches equilibrium with a
gas under an oxygen partial pressure of 10-6 Pascals (Pa) at
1,000K. In a gas with an oxygen partial pressure of higher than
10-6 Pascals (Pa), the reaction proceeds to form hematite. However,
when the partial pressure of oxygen is lower than this level,
hematite is decomposed and magnetite is formed; in other words,
hematite is reduced to magnetite.
Generally, with metal oxides, when the temperature increases,
the negative absolute value of the Gibb's energy of formation
will be smaller, which makes the oxides unstable and easy to
reduce. Similarly, the degree of stability of various oxides
can be compared by this figure. For example, the oxides of iron
are progressively less likely to be thermodynamically reduced
in the order of hematite, magnetite, wustite (FeO). Under the
conditions for wustite to be reduced, neither alumina nor the
oxides of titania are reduced, while the oxides of copper and
nickel are reduced.
The conditions for the formation of sulfides can also be discussed
by using diagrams for the Gibb's energy of formation, temperature,
and sulfur gas partial pressure of each reaction. When considering
the desulfurization in iron and steel making, it is necessary
to examine the Ellingham diagram in this manner. |
|
 |
 |
 |
|
|