When steel is heat treated, changes
occur not only to the crystal structure and grain size but also
in the state of the foreign atoms present in the steel. The minimum
equilibrium concentration of a foreign atom at which the atom
precipitates is defined as solubility limit. The foreign atoms
form solid solution when the concentration of the atom is less
than the solubility limit, and precipitate as a compound when
the solubility limit is exceeded. Solubility limit is determined
by the thermodynamic properties of entities which react to each
other to form precipitates. When interaction between the entities
is affirmative and Gibb's energy of the precipitate formation
is negatively large, precipitates are formed even at low concentrations
of the entities.
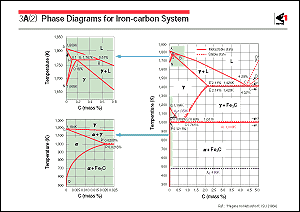
The figure shows an iron-carbon phase diagram, which is the most
fundamental for steel, showing how the transformation temperature
or solubility limit depends upon the carbon content. In the heat
treatment of low-carbon steel, the line segment PQ, which represents
the solubility limit of carbon in 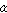 -ferrite, is
important. The solubility limit represented by PQ increases as
the temperature increases. Therefore, if, on heating, the solubility
limit increases and, subsequently, exceeds the carbon concentration
of the steel, all the carbides that have been precipitated will
decompose and dissolve. Precipitation will occur again when the
solubility limit decreases as the steel cools. -ferrite, is
important. The solubility limit represented by PQ increases as
the temperature increases. Therefore, if, on heating, the solubility
limit increases and, subsequently, exceeds the carbon concentration
of the steel, all the carbides that have been precipitated will
decompose and dissolve. Precipitation will occur again when the
solubility limit decreases as the steel cools.
Equilibrium theories based on thermodynamics deal with the stable
crystal structure and the state of foreign atoms. However, structures
formed in practice by heat treatment are not determined solely
by equilibrium theory. This is illustrated by the fact that the
carbon in the steel is precipitated not as graphite (thermodynamically
stable phase), but as the metastable cementite phase (Fe3C).
For graphite to precipitate, it would be necessary to achieve
complete diffusion of the carbon atoms to bring about the change
predicted by equilibrium theory. When such diffusion is not achieved,
for example in rapid cooling, the change is suspended while in
progress. On the other hand, plastic deformation accelerates
precipitation by increasing the precipitation sites available
and by promoting diffusion. By making use of these phenomena,
different structures can be obtained in steel of the same composition
by control of the crystal structure and the size and distribution
of the precipitated particles.
Fine precipitates induce strain in the surrounding crystal lattice
of iron, and consequently provide great resistance to dislocation
movements and increase the strength, even though they are present
in only minute amounts. Hence, elements which cause dissolution
and precipitation within the temperature range of heat treatment
and hot working are suitable for the formation of fine precipitates.
Typical elements like niobium and vanadium result in the formation
of carbonitrides. When steels containing these elements are hot
rolled, thermo-mechanical control processes are used practically
to increase strength by precipitating fine particles and by refining
the crystal grains, which is accomplished by controlling the
conditions for rolling and cooling. Thus, the structure of such
steels can be changed considerably by the heat treatment applied.
This makes it possible to produce steel materials with diverse
properties, and thus to select the properties suitable for specific
applications. |
|
 |
 |
 |
|
|