The steel products we encounter
everyday are polycrystalline materials consisting of many grains
of steel. Iron atoms arrange themselves regularly in one crystal,
and the direction of the arrangement of atoms differs among grains.
The diameter of an iron atom is 0.25 nanometers, while that of
a grain is usually 10 to 20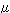 m. m.
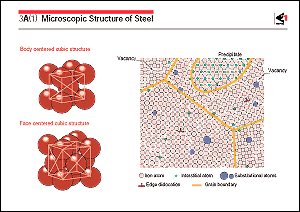
Iron atoms arrange themselves in one of two stable crystal structures
called the body-centered cubic structure and the face-centered
cubic structure. The body-centered cubic structure of iron, which
is called ferrite, is stable at (i) a temperature of 1,665K (1,392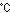 ) or above and (ii) at 1,184K (911 ) or above and (ii) at 1,184K (911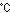 ) or below,
the crystal forms being referred to as ) or below,
the crystal forms being referred to as 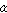 iron and a iron,
respectively. The face-centered cubic structure, which is called
austenite, is stable in a temperature range everywhere between
the above-mentioned two temperature ranges, and the iron of this
structure in this temperature range is called iron and a iron,
respectively. The face-centered cubic structure, which is called
austenite, is stable in a temperature range everywhere between
the above-mentioned two temperature ranges, and the iron of this
structure in this temperature range is called  iron. The phenomenon
by which a crystal structure changes to another due to a change
in temperature is referred to as a phase transformation. The
temperature at which this phenomenon occurs is called the transformation
temperature. The transformation temperature depends upon both
the nature and the amount of the alloying elements. iron. The phenomenon
by which a crystal structure changes to another due to a change
in temperature is referred to as a phase transformation. The
temperature at which this phenomenon occurs is called the transformation
temperature. The transformation temperature depends upon both
the nature and the amount of the alloying elements.
There are portions in actual grains where the regularity of the
positions of the iron atoms is lost, these portions being called
lattice defects. Particularly important lattice defects are (i)
"vacancies", which are point-like defects in which
an iron atom is missing at a lattice point, and (ii) "dislocations",
which are linear defects. Vacancies play an important role in
the diffusion of atoms, and plastic deformation occurs when dislocations
move. Foreign atoms, with a size different from that of iron
atoms, are present in a steel grain. These atoms exist in two
different forms, i.e., as a "solid solution", in which
they are present in the lattice structure of iron as shown in
the figure, and as a "precipitate", in which they form
another crystal structure and are present within the grain or
at the grain boundaries. Solid solutions are divided into interstitial
solid solutions and substitutional solid solutions. In the former
type, carbon, nitrogen, and other atoms much smaller than iron
atoms are located in the space between iron atoms. In the latter
type, atoms larger in size (aluminum, titanium), atoms that have
almost the same size (nickel, chromium), or atoms smaller in
size (silicon, phosphorous) than iron atoms, take the place of
some of the iron atoms.
A polycrystal is composed of many grains with different orientations.
Although a polycrystal usually has no orientation as a whole,
it can assume a texture that has many grains with specific orientations
under some working and heat-treatment conditions. A grain boundary
has excess energy; therefore, when it becomes possible for atoms
to move, a change occurs in such a manner that the area of the
grain boundary decreases; that is, grain growth occurs. The smaller
the grain size, the higher the strength and toughness. In other
words, the smaller, the grain size, the better. It is therefore
necessary to reduce the size of grains. Grains can be newly generated
by the two mechanisms of transformation and recrystallization.
Transformation was discussed above. Recrystallization is the
phenomenon in which, when a material is heated after being worked
beyond its critical strain, the strain energy accumulated by
working is released by diffusion which rearranges the position
of the atoms, and new grains are formed. Thus, grain refinement
is achieved by utilizing these mechanisms. |
|
 |
 |
 |
|
|