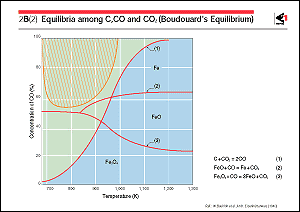
When reducing iron ore in the form
of iron oxide by using solid carbon as the reducing agent, the
carbon is oxidized into carbon monoxide and carbon dioxide.
The chemical reaction represented by Eq. 1 in the figure occurs
among solid carbon, carbon monoxide and carbon dioxide.
Curve 1 in the figure shows the equilibrium relationship for
this reaction, which is called Boudouard's equilibrium. When
the gas concentration of carbon monoxide is below this curve,
the reaction proceeds in the direction for forming carbon monoxide,
this being called the carbon solution reaction or solution-loss
reaction. In the neighborhood of 1,200K, carbon dioxide that
has been formed by the reduction of iron oxide is changed into
carbon monoxide by this reaction, making it possible to maintain
the reducing capability of the gas. On the other hand, when
the gas concentration of carbon monoxide is in the region above
curve 1, a carbon deposition reaction occurs according to equilibrium
theory; that is, carbon monoxide is dissociated into carbon dioxide
and carbon, and carbon is deposited. However, due to its extremely
slow speed, this reaction does not practically proceed at lower
temperatures and low carbon monoxide concentrations. Carbon
deposition actually occurs in the region where metallic iron
coexists to provide strong catalytic action, and in the region
of higher temperature and high carbon monoxide concentration.
This region corresponds to the shaded part in the drawing.
The figure also shows the regions of magnetite, wustite, and
iron metal that coexist in a stable manner with carbon monoxide
and carbon dioxide. It also shows that, at temperatures above
1,000K, reduction from magnetite to metallic iron can be achieved
with a composition at which carbon monoxide and carbon dioxide
are in equilibrium with each other.
Examination of the foregoing equilibrium theory makes it possible
to decide whether a desirable reaction is possible and which
conditions need to be met to obtain such a reaction. For practical
control of a reaction, however, the mechanism that controls the
reaction rate should be clarified and the heat and mass transfer
should be analyzed on the basis of reaction rate theory and transport
phenomena. |
|
 |
 |
 |
|
|